Sources of Morbidity and Mortality From Conventional Machines
In 1997, Caplan documented the causes of death and brain damage related to anesthesia gas delivery equipment.1 Of 3,791 closed anesthesia claims (1962-1991), only 72 were related to the gas delivery equipment, and the frequency of claims had decreased from 2.2% to 1.2% since 1985. Pure equipment failure was rare, with the rate of misuse three times higher. However, in 76% of claims, death or brain damage occurred. Caplan determined that better monitoring could have prevented injury in 78%. Could improvements in modern machines reduce these injuries further? Would any improvements be offset by generating new problems?
The breathing circuit was, by far, the major culprit (39%), causing a 70% incidence of death or brain damage. A misconnection or disconnection, most frequently, leads to hypoventilation or barotrauma. Next, the vaporizer (21%) contributed to an overdose, or to cases of awareness, but gas analysis or cerebral monitoring might have prevented these problems. The ventilator (17%) was not activated correctly, or it delivered an excessive tidal volume (Vt) or inspiratory pressure (Pi). High-pressure gas supplies (11%) injured patients if connected directly to the patient’s respiratory system, without appropriate decompression. The remainder of the gas machine was infrequently associated with injury (7%). Most importantly, human misuse was 3 times more common than failure of equipment.
Limitations of Conventional Anesthesia Machines
Definitions. For the purposes of this article, “Conventional” anesthesia machines include machines such as the Ohmeda2 Modulus series and Excel, and the North American Dräger3 Narkomed series and the Narkomed GS. Other brands share similar features. “Modern” machines, as discussed here, include the Datex-Ohmeda:2 Aestiva/5 (assuming 7900 ventilator), Anesthesia Delivery Unit (ADU) (assuming S/5 monitor system), and the Dräger Medical:3 Fabius GS v1.3, Julian, and Narkomed 6400 (all are abbreviated below). The author regrets that not all of the new machines could be included within this discussion.
Conventional anesthesia machines have many external connections. Despite standard-size hose fittings,4 multiple accessible connections are subject to disconnection or misconnection, kinking, omission, or obstruction. The morbidity from such events is determined by the location and function of the component. Connections of concern included those between breathing hoses, breathing system outlets, ventilator hoses, bellows, reservoir bag and arm, common gas outlet, fresh gas hose, unidirectional valves and domes, oxygen analyzer, airway pressure gauges, spirometer, PEEP valve, ventilator drive gas hose, ventilator relief valve, scavenger hoses, APL valve, scavenger interface connections, and suction lines.
Protection against barotrauma. Conventional machines incorporate pressure limiters in the mechanical breathing circuit, but some require a manual preset to maintain pressures below clinical extremes. Others will only generate an alarm when the preset value is exceeded. Patients could be at risk from activating the oxygen flush during inspiration, adding 500-800 ml/sec to Vt. When activated, a true limiter will end the inspiratory (I)-phase, or it will complete the I-phase at the pressure limited value, perhaps limiting Vt. Pressure sensors should be located as close to the patient’s trachea as possible, to avoid misleading airway pressure (Paw) data; many are not. Some APL valves are designed as variable resistors, instead of regulators, causing increased pressure at greater flow. Conventional APL valves cannot be fully opened instantly. Some conventional ventilators do not offer pressure controlled ventilation (PCV), while others might require manual adjustments to achieve PCV. Ventilators that deliver a metered volume of drive gas, without fully collapsing the bellows, could “stack” a breath on a patient breathing spontaneously; conventional systems do not have synchronized intermittent mandatory ventilation (SIMV) features. Standing bellows mandate 2-3 cm of PEEP, and scavenger systems with dirty valves could increase or decrease airway pressure.
Vaporizer risks. Variable bypass vaporizers are either fixed-mounted or removable. If tilted, liquid agent could enter the bypass chamber, vaporize, and then deliver an overdose of agent to the circuit. Inadequate volatile agent could be delivered if there is a leak around the mounting o-rings, which must be determined properly during pre-use checkout.5
Advanced ventilation features. Conventional anesthesia machines may not have integrated, variable PEEP, inverse ratio ventilation (IRV), or PCV for patients with ARDS, or the sensitivity and responsiveness needed to ventilate neonates. Neither do conventional ventilators offer pressure support ventilation (PSV) or SIMV for weaning patients from the ventilator.
Inaccurate delivery of set tidal volume. It is possible for the user to improperly set the inspiratory flow or time, such that the standing bellows of the, for example, Narkomed AV-2+ ventilator fails to descend completely. A potentially large percentage of the bellows volume is lost into the breathing circuit, secondary to compliance (e.g., ~5 ml/cm H2O) and compression (~3%). Leaks and sampled gas flow will further reduce desired Vt. Alternatively, the contribution of fresh gas (FG) to the I-phase might significantly increase Vt and Paw, similar to, but of less magnitude than the actuation of oxygen flush. Combined, these discrepancies significantly alter Vt. Furthermore, the spirometer will falsely report Vt, because it includes the breathing hose compliance. During volume controlled ventilation (VCV) of neonates or children, these discrepancies may constitute an overwhelming percentage of the desired Vt. Conventional ventilators typically require a two-step, mechanical/electrical conversion from manual ventilation; human error can leave the patient apneic.
Automated checkout. Conventional machines are manually inspected, often inaccurately, according to some variant of the 1996 FDA recommended checkout procedures. Clinicians fail to check their equipment thoroughly, are often not successful in detecting machine faults, or they don’t check their machines at all.6,7 Furthermore, in one study, clinicians’ knowledge of anesthesia machine structure and function did not correlate with their ability to detect preset machine flaws. Despite extensive instruction, anesthesia residents, at best, could only perform 81% of a checkout procedure.7
Low-flow adaptation. Concern about environmental contamination and waste of expensive volatile agents has created the desire for minimal (<0.5 lpm) or low flow (<1.0 lpm) anesthesia.8 A small volume of the breathing circuit would contribute to a faster change of inspired anesthetic concentrations (smaller time constant); conventional circuits are large (>6 l). A low-flow system should not have any leaks, which accumulate over multiple connections. Gas analyzer extraction should be returned to the circuit, yet it is not. At metabolic fresh gas flow (FGF) of oxygen, the inspired concentration (FiO2) might be well below the set ratio on the flow meter, and might vary over the period of inspiration. Monitoring FiO2 would be most accurate at the endotracheal tube, but is typically done within the inspiratory limb. Flow meters should be well calibrated at low FGF and retain their O2/N2O proportionating capability. Increased humidity in low flow circuits might cause more condensation. Suction at the scavenger system should not extract gas from the circuit. Spirometers should be accurate and corrected for changes in the volatile agent’s physical characteristics (density, thermal conductivity), if necessary. Large changes in FGF during induction and emergence should not alter set tidal volume, and there should be adequate warning against gradual reductions in peak airway pressure, entrainment of room air, or negative airway pressures.
Compressed medical gas consumption. Many facilities do not have piped medical gas, and must rely upon cylinders or no medical gas at all. Others leave their backup cylinders open (incorrectly), and these can be drained when wall supplies dip below the cylinder regulator pressure. Conventional ventilators are designed to consume an amount of drive gas roughly equivalent to the minute volume. Or they partially entrain room air through a venturi, and consume additional drive gas during an inspiratory plateau. Leaks and excessive FGF are further wasteful.
Solutions to Conventional Limitations
Reduced external connections. Systems which internalize connections should reduce the likelihood of misconnections, disconnections, or kinked connections. This is dramatically accomplished with internal modular (Aestiva/5) or manifold (Julian) components. However, kinking or disconnection of new APL and/or PEEP valve pilot lines may cause inappropriately high, or no pressure during controlled mechanical ventilation (CMV), depending on the timing of the event. A similar situation has occurred with a conventional machine.9
PEEP and scavenging. Electronically controlled PEEP is not prone to misconnection or imprecise settings. The piston (6400 and Fabius GS) and hanging bellows (Julian) ventilators eliminate undesired PEEP. The Aestiva/5, ADU, and Julian apply PEEP to the entire system, whereas the 6400 and Fabius GS only pressurize the I and E (expiratory) hoses. The scavenger system of Aestiva/5 and Julian must handle the ventilator drive gas in addition to ventilator relief gas, thus increasing the chance of undesired PEEP if suction were not properly applied. It is easier to set the suction on valveless “open” scavenger systems than it is to balance the proper amount of suction in older systems. All must have an adjustable pressure limiting device associated with the ventilator (ASTM F1850-00; 51.9.3.3.1).4
High pressure management. Early versions of the Aestiva had O2 cylinder regulators set to ~100 psig, thus draining the cylinder if it was left open with a 50 psig wall pressure. Later versions are set conventionally lower than wall, according to ASTM F1850-00: 62.2.4 The “fail-safes” have been eliminated from the air supply lines (except the 6400), such that medical air could be delivered through the vaporizer and into the circuit after complete loss of oxygen pressure.
Vaporization of anesthetic gas. The ADU uniquely features electronic control and measurement of vaporization, and eliminates the need for multiple vaporizers. Color-coded, magnetically labeled cassettes store each agent. Volatile agent delivery is reported to the Information Management System (IMS). Furthermore, the ADU uses these data 1) to make compensatory adjustments in N2O to maintain the desired FiO2, 2) to reduce the ventilator’s drive gas to maintain desired tidal volume, and 3) to report a calculated FiO2. Other systems do not yet calculate agent delivery, despite knowing the electronic FGF and the concentration of volatile agent. The cassettes have overfill protection, are automatically leak-tested during checkout, and have a check valve to prevent liquid from entering the bypass circuit. The desflurane cassette is warmed by heat from the CPU, which controls the flow of carrier gas into the cassette based upon the agent, its splitting ratio, selected concentration, FGF, and temperature. FGF, bypass flow, and flow emanating from the cassette are measured; agent and total flows are calculated. New “conventional” vaporizers offer keyed filling ports, and a new mechanism to prevent the escape of liquid through the vent hole, or into the bypass chamber when the vaporizer is tilted (Dräger Vapor 2000). The mechanism is active when the vaporizer is placed in the “T” position for removal or transport.
Ventilator pistons reduce medical gas consumption. Figure 1 and Figure 2 below are the author’s representation of the Fabius GS and 6400, respectively, which incorporate a piston instead of a bellows. These pistons are driven by highly refined mechanisms, at constant stroke velocity for VCV, or decreasing velocity (at set Paw) for PCV. The lung must be protected against suction from the downstroke of a rigid piston. The piston extracts gas from the reservoir (bag), which also serves as the decoupling reservoir for FGF during inspiration. Fabius GS fills the bag by first opening the exhalation valve/PEEP (EV/P) at end inspiration, whereas the 6400 first opens the DV. If there is inadequate gas in the reservoir, the Fabius GS piston entrains room air at -3.0 cm H2O from the cylinder’s negative relief valve. The 6400 will not (some versions may) entrain room air, but rather will stop the downstroke of the piston, and inform the user. Pistons can also eliminate all residual “bellows” volume at end inspiration for VCV, by traveling to top-dead-center; it begins at the bottom in PCV. The Fabius GS piston is isolated from patient/gas by a membrane; the 6400 is exposed. Piston Vt can be reduced to 20 and 10 ml. Fabius can operate without compressed gas, but the 6400 requires oxygen pressure to inflate the membrane surrounding the perimeter of the piston. Both systems offer many advanced ventilation features described above.
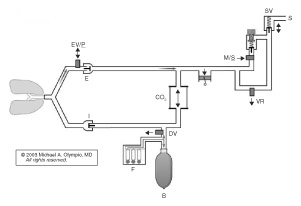
Figure 1. Fabius GS demonstrates the path of fresh gas (F) (and O2 flush) into the reservoir bag during upstroke of the piston. Note the requirement here for a mechanical decoupling valve (DV) and electronic exhalation/PEEP/Plimit valve (EV/P). A man/spont (M/S) valve is electronically opened during CMV, so that excess gas can escape through the low pressure scavenger valve (SV), which also allows preferential filling of the reservoir bag during the I-phase. Note the minimal pressurized volume of this circuit during the I-phase, and the unique capability to ventilate the patient with no reservoir bag, no absorber, and no compressed gas, using only room air!
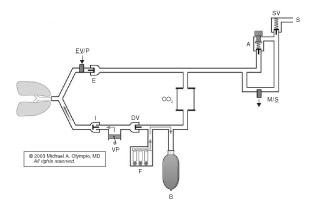
Figure 2. Narkomed 6400 demonstrates a conventional location for the ventilator and the relocation of the reservoir bag to the inspiratory limb to capture FGF during inspiration by closing the DV. At the onset of exhalation (shown), the DV opens first, with filling of the reservoir bag as the patient exhales against the PEEP valve (EV/P). This is followed by piston retraction to set Vt, and finally by opening of the ventilator relief valve (VR) to allow the escape of excess gas.
Methods for accurate delivery of set Vt:
1) Fresh gas decoupling (FGD) implies that FG is not delivered to the patient during inspiration (absolute). Practically, it means that FGF does not contribute to Vt. FGD is accomplished in a variety of ways, all of which have increased the complexity of the circuits, valves, feedback, and control mechanisms: a) Aestiva/5 delivers the first metered volume of drive gas and measures the delivered volume at the I-valve. Then it adjusts subsequent drive gas volumes, thereby eliminating proximal compliance, compression, and FG contributions to Vt. This mechanism requires several breaths to equilibrate and would not prevent an immediate “first breath” increase in Vt and Paw from higher FGF. b) The ADU preemptively measures FG and volatile agent flow, subtracting this amount from the metered drive gas before it is ever delivered, thus “decoupling” the FGF. Realize that neither of these mechanisms would immediately protect against O2 flush during inspiration. c) New breathing circuit designs and valves in the Fabius GS and 6400 accomplish (absolute) FGD, by re-direction of FGF into a reservoir during inspiration. A series of electronically controlled valves must work in concert with the ventilatory phase to accomplish this decoupling.
2) A second method for accurate delivery of Vt is compliance compensation, to replace volume lost to the breathing hoses and/or circuit. Internal compliance is very low, is constant, and could be used as a default value. However, the ADU, Fabius, Julian, and 6400 all measure the total compliance (with attached hoses) during checkout and use this data to incrementally adjust piston or bellows excursion, according to generated Paw. The volume trapped within the breathing hose compliance will pass through the spirometer, adding a “false” volume to Vt. The Fabius GS and 6400 display the net volume after subtraction of this “false” volume.
3) Leaks are measured and reported by all (except Aestiva/5) but are never automatically compensated. The 6400 and Julian re-measure periodically during CMV. Gas analyzer suction could be retrievable and returned to the system, but this requires FDA approval.
4) Location of the PEEP valve could affect delivered Vt; however, with compliance compensation these effects may not be significant.
5) Electronic settings of the new ventilators should reduce previous user errors with incorrect or imprecise settings.
6) One-step activation of CMV in the Aestiva/5 and ADU might prevent previous failures to initiate CMV; the others require an additional confirmatory step.
Automated checkout and monitoring. A multitude of surveillance alarms and automated checkout procedures are associated with the increasing complexity of these systems, since the user cannot determine a problem or test for tolerances by conventional checkout methods. For example, the Fabius GS can detect and report an incompetent exhalation valve because the exhalation flow sensor “knows” there should not be flow during the inspiratory phase. Computer circuit loops, flow meters, pressure sensors, piston drive gears, coordination and operation of valves, compliance compensation, fresh gas control, display of ventilation, and prevention of airway pressure anomalies are just a few of the systems that are critical to proper function. Despite these automated checkout procedures, not every fault, obstruction, crossed connection (e.g., between the inspiratory limb and hose to the reservoir bag, in the ADU) or disconnection may be detected. Most important is the immediate availability of functional, tested back-up ventilation equipment. Because of conventional concerns (leaks) in the low pressure system,5 the user must understand the various requirements for low pressure checkout in these new systems, and whether to open, close, or remove the vaporizers or cassettes, during testing: the ADU actually does pressurize the Aladin™ cassette, thus checking it for leaks; the Dräger Vapor 2000 at “0” has fixed pins that depress the valves in the backbar, thus checking the bypass circuit, but not the vaporization/fill chamber for leaks. New workstation standards (ASTM F1850-00: 51.10.5.1)4 incorporate capnography, which can be used to detect incompetent I and E valves. Some automated tests can be bypassed several times, and eventually will not allow use of the machine (e.g., 6400) until retested.
Streamlined communication with Information Management Systems (IMS). This increasing complexity of machines is consistent with, and contributes to the digital information revolution. Integrated physiological and respiratory monitoring data, combined with the digital FG and volatile agent flow data, are all easily and neatly sent to an IMS, sold as options by these companies, and exceed ASTM standards.4 The data now extend beyond the anesthetic record, and may communicate with other demographic and financial records. The Anesthesia Patient Safety Foundation supports ongoing methods to standardize this flow of information, through the Data Dictionary Task Force.10
Dependence upon electricity. All of these systems have battery backup and some warn of impending power loss, suggesting a switch to manual ventilation. In the event of a dead battery, all can deliver oxygen, but not measure it electronically, and they all allow manual or spontaneous ventilation with manual control of the APL valve. ADU cannot deliver volatile agent; Julian terminates all FGF, requiring the user to open a needle valve for O2, which could still flow through the vaporizer; ADU terminates delivery of N2O but allows air substitution; Fabius GS and ADU provide a single tube flow meter to estimate combined FGF; all will lose PEEP.
| Anasthesia Machine Comparisons | Narkomed AV2+ | Ohmeda 7800 | 6400 | Julian | Fabius GS 1.3 | Aestiva/5 | ADU |
|
|
|||||||
| Does fresh gas flow increase Vt? | Yes | Yes | No | No | No | Initially | No |
| Is the pre-use system leakage measured? | No | No | Yes | Yes | Yes | No | Yes |
| Is there compensation for a proximal leak? | No | No | No | No | No | Yes | No |
| Is leakage measured during the case? | No | No | Yes | Yes | No | No | No |
| Is the hose compliance compensated? | No | No | Yes | Yes | Yes | No | Yes |
| Is the system compliance compensated? | No | No | Yes | Yes | Yes | Yes | Yes |
| Is the reported exhaled Vt adjusted for hose? | No | No | Yes | No | Yes | No | No |
| The fresh gas inflow is distal to what? | Absorber | Absorber | Absorber | Mid-absorber | Absorber | Absorber | INSP valve |
| The fresh gas inflow is proximal to what? | INSP valve | INSP valve | Decoupling valve | Mid-absorber | Decoupling valve | INSP valve | Y-piece |
| At low FGF, exh, what gas fills the reservoir bag? | Exhaled | Exhaled | Scrubbed | Exhaled | Scrubbed | Exhaled | Exhaled |
| What is the mechanism of VCV? | Mech. limit | Metered | Displacement | Metered | Displacement | Metered/servo | Metered/calc |
| How is PCV controlled? | P-limited | None | Flow/p-limited | Flow/p-limited | Flow/p-limited | P-limited | Flow/p-limited |
| Is FiO2 compensated for volatile agent? | No | No | No | No | No | No | Yes |
| Is the SIMV mode offered? | No | No | Yes | No | No | No | Yes |
| What is the specified minimum Vt? | 18 | 10 | 50 | 20 | 20 | 20 | |
| How is fresh gas flow controlled? | Needle valve | Needle valve | Needle valve | Digital control | Needle valve | Needle valve | Needle valve |
| How is fresh gas flow measured? | Flow tubes | Flow tubes | Flow tubes | Electronic | Electronic | Flow tubes | Electronic |
| Is there a backup flow tube? | N/A | N/A | N/A | No | Yes | N/A | Yes |
| Is there integrated capnography? | No | No | Yes | Yes | No | No | Yes |
| Is there integrated anesthetic gas monitoring? | No | No | Yes | Yes | No | No | Yes |
| What is the effect of lost oxygen pressure on FGF? | No FGF | No FGF | No FGF | Auto air on | Air available | Air available | Air available |
| Return of sampled gas to circuit? | No | No | No | No | No | No | Yes |
| Is there a mechanical Paw gauge? | Yes | Yes | No | No | Yes | Yes | No |
| Can you remove the absorber during VCV? | No | No | No | No | Yes | No | Yes |
| Likely to entrain room air with a circuit leak? | No | No | Yes | Yes | Yes | No | No |
| Might it entrain room air with inadequate FGF? | No | No | No (version) | No | Yes | No | No |
| Can you provide CMV without any FG pressure? | No | No | No | No | Yes | No | No |
| Effect of O2 flush during VCV inspiration? | >Vt, held at P-limit | >Vt, end at P-limit | None | >Vt, held at P-limit | None | >Vt, end at P-limit | >Vt, end at P-rel |
| Can it provide zero PEEP in CMV? | No | No | Yes | Yes | Yes | No | No |
| How do you convert from VCV to PCV? | Mechanical | N/A | Automatic | Electronic reset | Automatic | Electronic reset | Electronic reset |
| Unique aspect of the O2 cylinder regulator? | None | None | None | Electronic | None | Was 97 psi | None |
| Is the failsafe integrated with the ratio controller? | No | No | No | Yes-electronic | Yes-pneumatic | No | Yes-electronic |
| How can you find a low pressure (vaporizor) leak? | Positive pressure | Negative pressure | Auto, vap open | Auto, vap open | Auto, vap open | Negative pressure | Automatic |
| # of valves “controlled” in breathing circuit? | None | None | 4 | 2 | 2 | 1 | 1 |
| Does machine scavenge ventilator drive gas? | No | No | N/A | Yes | N/A | Yes | No |
New Concerns Regarding Modern Machines
This new generation of advanced anesthesia machines raises new questions. Will a sudden increase in compliance (as with the opening of a tense abdomen in a neonate on VCV) lead to sudden hyperinflation of the lung? What if compliance is measured in the adult circuit, and then a pediatric circuit is added for neonatal anesthesia? Will that infant receive too much volume? New machines still do not warn the user of consumption of oxygen from an open cylinder, as wall pressure drops below regulator pressure. Why do tilt-proof vaporizers allow deactivation of the safety device, once it is removed from the machine? Will new circuit designs cause clinically significant increases (or decreases) in time constants, when changing anesthetic depth? If a ventilator compensates for a proximal leak, what would be the effect on the patient if the leak was suddenly sealed? Will exposed pilot lines to critical valves become disconnected or kinked during CMV, and what might happen to the patient? What types of flammable substances might enter the breathing circuit and cause a fire secondary to a hot-wire spirometer? With an obstruction, will the clinician be fooled by artificial ventilator sounds into thinking that the patient is being ventilated when he or she is not? What will be the best monitor of ventilation in a hidden-piston ventilator? Will the automated checkout procedures prevent emergent and rapid initiation of the machine? Can a machine have a clinically significant latent failure after passing the automated checkout? Will numerous external connections in some of these machines lead to a misconnection or disconnection? Despite capnometry, will a hanging bellows mislead clinicians into thinking they are ventilating the patient when they are not? Will a mixture of modern and conventional machines confuse the clinician? Will single step activation of mechanical ventilation cause the clinician to forget the second step in other systems? Are we still able to pour the wrong agent into a vaporizer? Will breath stacking occur with SIMV? New solutions to old problems might raise new problems. We must remain educated, prepared, and vigilant.
Dr. Olympio is Professor of Anesthesiology, Vice Chair for Education and Director of the Patient Simulation Laboratory at Wake Forest University School of Medicine in Winston-Salem, NC.
References
- Caplan RA, Vistica MF, Posner KL, Cheney FW. Adverse anesthetic outcomes arising from gas delivery equipment: a closed claims analysis. Anesthesiology 1997;87:741-8.
- Datex-Ohmeda, Inc., Madison, WI, USA (including operator and/or service manuals for the above products, and personal communications).
- Dräger Medical, Inc., Telford, PA, USA (including operator and/or service manuals for the above products, and personal communications).
- Standard Specification for: Conical Fittings (F1054-01), Minimum Performance and Safety Requirements… (F1208-89(200)e1)), and Particular Requirements for Anesthesia Workstations… (F1850-00). American Society for Testing and Materials, F-29 Subcommittee.
- Myers JA, Good ML, Andrews JJ. Comparison of tests for detecting leaks in the low-pressure system of anesthesia gas machines. Anesth Analg 1997;84:179-84.
- March MG, Crowley JJ. An evaluation of anesthesiologists’ present checkout methods and the validity of the FDA checklist. Anesthesiology 1991;75:724-9.
- Olympio MA, Goldstein MM, Mathes DD. Instructional review improves performance of anesthesia apparatus checkout procedures. Anesth Analg 1996;83:618-22.
- Baum MA. Low Flow Anaesthesia: The Theory and Practice of Low Flow, Minimal Flow and Closed System Anaesthesia. Butterworth-Heinemann, Oxford, 1996.
- Eisenkraft JB. Potential for barotrauma or hypoventilation with the Dräger AV-E ventilator. J Clin Anesth 1989;1:452-6.
- O’Reilly M. APSF conference highlights practical aspects of anesthesia information systems. APSF Newsletter 2002-03;17:53.

