History of Coronary Stents
Since the introduction of percutaneous transluminal coronary angioplasty (PTCA) by Gruntzig in 1977, major advancements have been made in the clinical practice of percutaneous coronary intervention (PCI). Puel and Sigwart, in 1986, deployed the first coronary stent to act as a scaffold, thus 1) preventing vessel closure during PTCA, and 2) reducing the incidence of angiographic restenosis, which had an occurrence rate of 30-40%.1 By 1999, stenting composed 84.2% of all PCIs.1 Despite the widespread use of these devices, bare metal stents (BMS) have been associated with a 20-30% restenosis rate requiring reintervention.2,3 Restenosis occurs as a result of neointimal hyperplasia—growth of scar tissue within the stent—due to the proliferation and migration of vascular smooth muscle cells. This phenomenon is clinically evident within the first 6-9 months after stent placement, and occurs in response to strut-associated injury and inflammation.2
In addition to restenosis, PTCA and BMS implantation cause exaggerated endothelial injury and inflammation, rendering both the stent and vessel highly thrombogenic.4,5 A fibrinogen layer covers the stent surface, further inducing platelet activation and thrombosis. Adjunctive anti-platelet medication is crucial in preventing local coronary thrombosis, myocardial infarction (MI), and death.5 Current recommendations for patients with BMS include dual anti-platelet therapy with aspirin and clopidogrel, which are continued for 6 weeks to allow complete endothelialization of BMS.6 Wilson et al. in 2002 reported similar findings in patients who underwent noncardiac surgery.7 The incidence of MI and death were significantly lower among patients who underwent surgery after their 6-week course of aspirin and clopidogrel were completed.
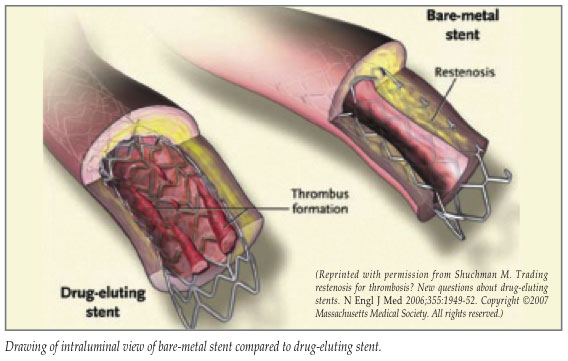
In 2001, drug-eluting stents (DES) were introduced as a strategy to minimize restenosis and requirement for reintervention. The currently available polymer-coated stents contain antiproliferative agents which elute locally in the implanted coronary artery to prevent neointimal hyperplasia. Initial animal studies demonstrated a clear benefit over BMS (4-6% restenosis versus 20-30%), and early clinical trials further supported this.2,8 In addition, at 2-year follow-up using both angiography and ultrasound, the clinical safety of DES was further established with minimal late lumen loss observed.9 A recent pooled analysis demonstrated a 74% reduction in the risk of target lesion revascularization for both sirolimus-eluting stents (SES) and paclitaxel-eluting stents (PES) compared to BMS.10 At present, 90% of all stents placed in the United States and Europe are DES.
Despite the enthusiasm that resulted with the advent of DES, incomplete endothelialization and stent thrombosis continue to plague these devices. Initial animal studies demonstrated complete endothelialization with BMS at 28 days, whereas DES uniformly showed incomplete healing at 180 days.11 Based on early observations in both animal and human studies, it was recommended that patients with DES receive dual anti-platelet therapy with aspirin and clopidogrel for at least 3-12 months, followed by life-long aspirin therapy, depending on the stent placed and the pre-existing comorbidities which further increase the risk of stent thrombosis.12,13 Despite this regimen, late stent thrombosis (LST)—defined as occurring >30 days post-stent insertion—remains a significant complication in patients with DES. Late stent thrombosis carries a 45% mortality rate.14,15 It presents as an ST-segment elevation myocardial infarction (STEMI) or sudden death. Late stent thrombosis has been documented in both clinical and autopsy studies in patients as far as 4 years after stent insertion.14-16 Further, LST is associated with the 1) discontinuation of clopidogrel +/- aspirin, 2) stable aspirin monotherapy, or 3) a hypersensitivity reaction to the stent polymer, or to the antiproliferative agent (sirolimus vs. paclitaxel).10-15 A recently published study reported that patients with DES implanted had significantly increased rates of death when clopidogrel was discontinued at 6-, 12-, and 24-months when compared to patients who remained on this therapy at the same time intervals.16
Coronary Stents and Surgery
Patients with DES pose a particular dilemma in the perioperative period. Current recommendations include delaying noncardiac surgery until the course of dual anti-platelet therapy is complete. Based on current clinical and autopsy findings, it is unclear how long dual anti-platelet therapy must continue to prevent LST.13-18 It is clear, though, that patients must remain on aspirin forever. This scenario is particularly challenging to us as anesthesia providers, as there are no guidelines currently to manage these patients perioperatively. The perioperative period is especially problematic because 1) surgery induces a hypercoagulable state; 2) surgeons often stop aspirin +/- clopidogrel preoperatively to minimize the risk of surgical bleeding, but without consulting their patients’ cardiologists; and 3) there is a high likelihood that the DES are not yet endothelialized. Thus, each DES patient, if stent thrombosis occurs, has a 45% chance of dying perioperatively. There are 3 points to consider: 1) transition of dual anti-platelet therapy in the perioperative period; 2) returning patients to their regimen as soon as possible postoperatively; 3) maintaining these patients on aspirin throughout the entire perioperative period, since perioperative STEMI and death have been associated with the discontinuation of aspirin in these patients.10
Our Current Approach to Perioperative Patients With Stents
We collaborated with the interventional cardiologists at Wake Forest University Health Sciences to develop a strategy to best manage these patients. Our protocol includes utilizing both eptifibatide (Integrilin, a GP IIb/IIIa inhibitor) and heparin as “bridging therapy” to prevent stent thrombosis in the perioperative period. Both medications are necessary in order to 1) prevent platelet activation and adhesion (eptifibatide) and 2) prevent thrombin formation (heparin), which again causes platelet activation and clot formation. Both eptifibatide and heparin have short half-lives, necessitating these drugs to be given as intravenous infusions. Further, both drugs can be stopped 6 hours prior to surgery with complete return of platelet function and coagulation. Cooperation between anesthesiology, cardiology, and surgery are of the utmost importance. The surgeon may elect to proceed with surgery while the patient remains on clopidogrel and aspirin. Alternatively, if the surgeon feels that perioperative clopidogrel will be deleterious in terms of increased surgical bleeding, then the following protocol will be instituted:
-
- The following information must be obtained from the patient’s cardiologist:
- Type(
s) of DES placed and date of procedure - any complexities associated with stent placement (bifurcations, coronary vessel diameter, total stent length)
- comorbidities: renal failure, diabetes, depressed ejection fraction.
- Type(
- Clopidogrel is discontinued 5 days prior to surgery (a cardiology consult should be obtained prior to discontinuation of clopidogrel).
- Aspirin must be continued throughout the perioperative period.
- The patient will be admitted to the appropriate surgical service 2 days prior to surgery to receive bridging therapy (eptifibatide and heparin) and prevent stent thrombosis.
- The bridging therapy will be initiated according to the paradigm shown in Table 1.
- IV eptifibatide and heparin infusions will be discontinued 6 hours prior to surgery to 1) facilitate normal intraoperative platelet function and coagulation, and 2) allow for regional anesthetic techniques to be performed preoperatively.
- Upon agreement between cardiology and surgery, clopidogrel/eptifibatide will be readministered as soon as possible postoperatively (preferably, the postoperative night):
- clopidogrel loading dose: 600 mg p.o.
- clopidogrel maintenance dose: 75 mg p.o. daily
- eptifibatide infusion will be restarted according to the above paradigm if clopidogrel cannot be reinitiated.
- The following information must be obtained from the patient’s cardiologist:

In conclusion, DES represents the most current therapy in interventional cardiology. However, late stent thrombosis is a major problem with these devices. In fact, the FDA has recently reviewed the safety of these devices, and new recommendations regarding dual anti-platelet therapy may be forthcoming. By utilizing a combination of eptifibatide, heparin, and aspirin, the risk of stent thrombosis will be markedly reduced in the perioperative period. However, we will continue to address and modify our therapeutic approach as the dynamic nature of this subject continues to evolve. This is an important patient safety issue because of the high mortality rate if stent thrombosis occurs.
Editor’s Note: While this issue of the APSF Newsletter was in production, a pre-publication scientific advisory from the American Heart Association was released electronically. This advisory addresses the issue of premature discontinuation of antiplatelet drugs in patients with drug-eluting stents, including patients presenting for non-cardiac surgery. The Advisory will be published in the February 13, 2007 issue of Circulation.
References
1. Serruys PW, Kutryk MJB, Ong ATL. Coronary-artery stents. N Engl J Med 2006;354:483-95.
2. Moliterno DJ. Healing Achilles—sirolimus versus paclitaxel. N Engl J Med 2005;353:724-6.
3. Arjomand H, Turi Z, McCormick D, et al. Percutaneous coronary intervention: historical perspectives, current status, and future direction. Am Heart J 2003;146:787-96.
4. Gawaz M, Neumann FJ, Ott I, et al. Platelet activation and coronary stent implantation. Effect of antithrombotic therapy. Circulation 1996;94:279-85.
5. Caramori PRA, Lima VC, Seidelin PH, et al. Long-term endothelial dysfunction after coronary stenting. J Am Coll Cardiol 1999;34:1675-9.
6. Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery-executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines On Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation 2002;105:1257-67.
7. Wilson SH, Fasseas P, Orford JL, et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol 2003;42:234-40.
8. Biondi-Zoccai GG, Agostoni P, Abbate A, et al. Adjusted indirect comparison of intracoronary drug-eluting stents: evidence from a metaanalysis of randomized bare-metal-stent-controlled trials. Int J Cardiol 2005;100:119-23.
9. Sousa JE, Costa MA, Sousa AG, et al. Two-year angio-graphic and intravascular ultrasound follow-up after implantation of sirolimus-eluting stents in human coronary arteries. Circulation 2003;107:381-3.
10. Hill RA, Dundar Y, Bakhai A, et al. Drug-eluting stents: an early systematic review to inform policy. Eur Heart J 2004;25:902-19.
11. Tsimikas S. Drug-eluting stents and late adverse clinical outcomes: lessons learned, lessons awaited. J Am Coll Cardiol 2006;47:2112-5.
12. Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous interventions. The task force for percutaneous coronary interventions of the European Society of Cardiology. Eur Heart J 2005;26:804-47.
13. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005;293:2126-30.
14. McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519-21.
15. Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans. J Am Coll Cardiol 2006;48:193-202.
16. Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 2006;297: epublished December 5, 2006. Available at: http://jama.ama-assn.org/cgi/reprint/ 297.2.joc60179v1. Accessed December 7, 2006.
17. Ong ATL, McFadden EP, Regar E, et al. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 2005;45:2088-92.
18. Ferrari E, Benhamou M, Cerboni P, et al. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol 2005;45:456-9.
Dr. Royster is Professor and Executive Vice Chair, Department of Anesthesiology, Dr. Newsome is Assistant Professor of Anesthesiology, Dr. Gandhi is Assistant Professor of Cardiology, and Dr. Kutcher is Associate Professor of Cardiology—all are faculty at Wake Forest University Health Sciences, in Winston-Salem, NC. Dr. Prielipp is the J.J. Buckley Professor and Chair, Department of Anesthesiology at the University of Minnesota in Minneapolis, MN and Chair of the APSF Committee on Education.


 Issue PDF
Issue PDF