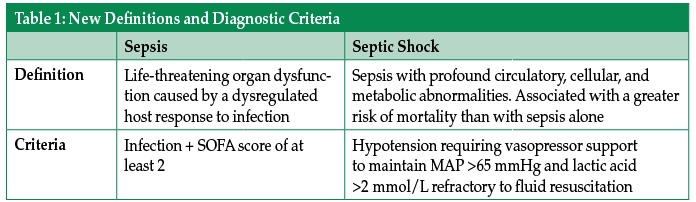
An experienced health care provider can identify the septic patient with barely a glance, but were you to ask them to define sepsis, many providers would struggle to provide a clear definition. This difficulty likely stems from a failure of understanding of the underlying pathophysiology of sepsis. A new consensus definition, released in early 2016, sought to more clearly define sepsis and septic shock.1 According to these new definitions, sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection, while septic shock is a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone.
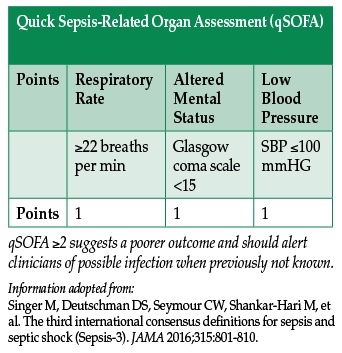 Previous guidelines used 4 criteria to identify patients with the systemic inflammatory response syndrome (SIRS), including temperature, heart rate, respiratory rate, and white blood cell count—measures that have been shown to be highly sensitive but lacking specificity, especially in the elderly.2 The new guidelines abandon these SIRS criteria. Instead, they focus on the Sequential Organ Failure Assessment (SOFA) score—a measure that determines the extent of a patient’s organ function or rate of failure (and incorporates a scoring system for respiratory, cardiovascular, hepatic, coagulation, renal, and neurological systems).3 The SOFA score has been associated with increased mortality in intensive care units.3 A score of 2 points or more above the patient’s baseline at the onset of sepsis has been associated with an in-hospital mortality of 10%.1 SOFA score may be useful to identify acutely ill patients coming to the operating room or other procedural areas under the care of an anesthesia provider.
Previous guidelines used 4 criteria to identify patients with the systemic inflammatory response syndrome (SIRS), including temperature, heart rate, respiratory rate, and white blood cell count—measures that have been shown to be highly sensitive but lacking specificity, especially in the elderly.2 The new guidelines abandon these SIRS criteria. Instead, they focus on the Sequential Organ Failure Assessment (SOFA) score—a measure that determines the extent of a patient’s organ function or rate of failure (and incorporates a scoring system for respiratory, cardiovascular, hepatic, coagulation, renal, and neurological systems).3 The SOFA score has been associated with increased mortality in intensive care units.3 A score of 2 points or more above the patient’s baseline at the onset of sepsis has been associated with an in-hospital mortality of 10%.1 SOFA score may be useful to identify acutely ill patients coming to the operating room or other procedural areas under the care of an anesthesia provider.
A new rapid, bedside tool to identify sepsis at presentation was proposed by the expert panel which released the new definition. The quickSOFA score (qSOFA) has 3 criteria—respiratory rate >22 bpm, altered mental status, and systolic blood pressure <100 mmHg. Using qSOFA, any provider may quickly identify upon initial evaluation any patient meeting at least 2 of the criteria as likely having sepsis, and initiate immediate appropriate therapy and further evaluation of organ dysfunction.4 This may prove to be useful in the emergency department and other ambulatory settings. However, further attempts at validating qSOFA are forthcoming.
The 2012 Surviving Sepsis Campaign guidelines for the management of severe sepsis outline and still remain the foundations of care—early recognition, source control, resuscitation, and timely antibiotic therapy.5 One recent study suggested that time to administration of appropriate antibiotic therapy may impact both ICU and hospital length of stay.6 In many septic patients, source control may require a trip to the operating room (OR), interventional radiology suite, or other procedural areas under the care of an anesthesia provider.
Resuscitation of the Septic Patient in the Operating Room
It is likely that the anesthesia provider will continue resuscitation efforts that have been ongoing in the ICU, Emergency Department (ED), or hospital floor in the OR. Care of the septic patient may require invasive monitoring, in addition to the standard monitors. An arterial line may serve as a reliable monitor of arterial blood pressure to guide resuscitation. Patients may require central venous access as well for administration of fluids when peripheral intravenous access is inadequate or for long-term administration of vasoactive medications.
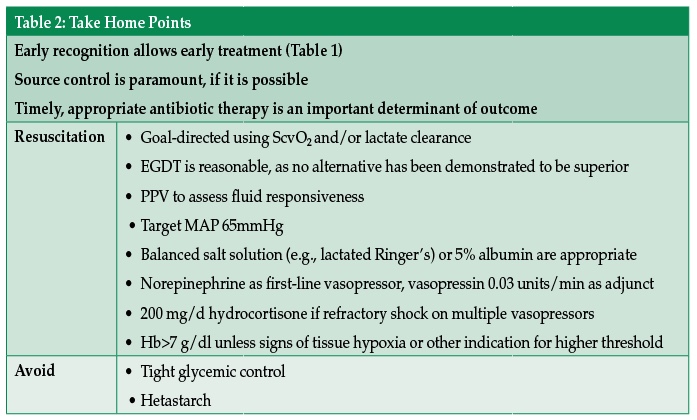
The identification of which patients will respond to volume resuscitation in sepsis is important. While central venous pressure (CVP) is a poor predictor of fluid responsiveness,7 it remains in widespread use as an indicator of volume status.8 Studies suggest that pulse pressure variation on an arterial line (PPV-variation in pulse pressure between inspiration and expiration with positive pressure ventilation) may be superior to central venous pressure as a predictor of volume responsiveness in septic patients, and may be used whenever clinical circumstances allow.9 However, PPV may be invalid in several scenarios, including but not limited to a non-sinus rhythm, low tidal volume ventilation, ventilator-patient dyssynchrony, altered chest wall or pulmonary compliance, pulmonary hypertension, elevated intra-abdominal pressure, or with an open chest.9,10In 2001, Rivers et al. published his landmark article and an algorithm for early goal-directed resuscitation (EGDT) of the septic patient using mean arterial pressure (MAP), CVP, and central venous oxygen saturation (ScvO2) to guide resuscitation within the first 6 hours of admission, primarily in the ED.11 This approach, quickly adopted by many providers, was recently compared to standard practice in a series of studies. While EGDT was not shown to be a superior approach to standard practice, it was not inferior.12-14 While consensus has not been reached on a universal set of hemodynamic goals to guide resuscitation of the septic patient, EGDT of patients with septic shock remains a reasonable algorithm to manage these patients, with or without invasive monitors. The question of which measures and what goals to use for titration are evolving, and will almost certainly be influenced by new expeditious tools that are developed to identify septic patients.
Maintaining Blood Pressure
Mean arterial pressure is a preferred choice as a parameter to monitor in the resuscitation of the septic patient.15 The Rivers trial among others somewhat arbitrarily chose a MAP of 65 mmHg as a target to maintain tissue perfusion. A more recent multicenter, randomized study comparing a low-MAP target (65-70 mmHg) to a high-MAP target (80-85 mmHg) in septic patients found no difference in mortality between the 2 groups.16
The maintenance of an adequate blood pressure will typically require some combination of fluid administration and vasoactive support. For the resuscitation of the septic patient, both crystalloid and colloid may be considered. Balanced salt solutions like Lactated Ringer’s or Plasma-Lyte may cause less acidemia and kidney injury than saline solutions in surgical patients,17 and are associated with lower in-hospital mortality in sepsis.18 Albumin has been shown to be non-inferior to, and possibly superior to, crystalloid for the resuscitation of the septic patient and particularly in the septic shock patient.19,20 However, its benefit should be weighed against the significant incurred cost. At present, starch solutions should be avoided for resuscitation in sepsis, as they may increase mortality, risk of acute kidney injury, and the need for renal replacement therapy.21
If fluid administration is not sufficient to maintain adequate blood pressure, norepinephrine may be considered as the vasopressor of choice. Norepinephrine has been associated with a lower mortality and lower risk of tachyarrhythmias than dopamine.22 Adding vasopressin to norepinephrine at a dose of 0.03 U/min can be considered as a catecholamine-sparing adjunct to norepinephrine, but has not shown to decrease mortality.23 If norepinephrine and vasopressin at maximal doses cannot adequately maintain MAP >65 mmHg, epinephrine may be added or substituted. Phenylephrine is typically a second- or third-line agent to maintain MAP in septic patients but can also be used in those patients with arrhythmogenic complications of catecholamines.5
Monitoring of Resuscitation
One method of estimating the adequacy of resuscitation is the measurement of central venous blood oxygen saturation (ScvO2). ScvO2 drawn from the sinoatrial junction, while not equivalent to mixed venous oxygen saturation (SvO2) drawn from the pulmonary artery, correlates well in the initial resuscitation period in sepsis.24,25 This correlation may become less consistent as early as 6 hours into resuscitation.26 In sepsis, ScvO2 is normally elevated well above baseline. In the Rivers EGDT trial, the protocol used a target ScvO2 of at least 70% to signify an adequate balance of oxygen delivery relative to utilization. Despite the use of ScvO2 in the Rivers trial, there is wide variability in the use of ScvO2 in the resuscitation of septic patients, largely due to the requirement for central venous access.27
An alternative to venous oxygen saturation for the evaluation of the circulation, and one that can be used in the absence of a central line, is serum lactate level and lactate clearance. By comparing the lactic acid level of 2 blood samples drawn at least 2 hours apart, the “lactate clearance” can be calculated. This difference can be used to assess the adequacy of resuscitation in septic patients. This method has been shown to be non-inferior to ScvO2 use, with a target decrease in lactate of at least 10%.28 The addition of lactate clearance to the traditional Surviving Sepsis Campaign bundle may lead to decreased mortality in sepsis patients.29
Transfusion of blood and the infusion of inotropes can also be used to both increase ScvO2 and decrease lactate levels. However, a recent multicenter randomized trial has subsequently shown that there is no benefit of using a transfusion threshold of 9 g/dl over a threshold of 7 g/dl in sepsis.30 Because most patients will have central venous saturations above 70%, it is relatively uncommon for septic patients to require or be treated with inotropes such as dobutamine.
Etomidate
Although induction with etomidate has minimal cardiovascular depression relative to other induction agents, it suppresses adrenal steroidogenesis by directly inhibiting 11ß-hydroxylase.31 The administration of a single dose of etomidate for intubation in patients with sepsis increases the risk of adrenal insufficiency, and possibly the risk of mortality as well.32,33 Therefore, etomidate should be used with caution in this patient population.
Steroid Replacement
Early steroid replacement has not been demonstrated to be beneficial for all patients in septic shock.34 Patients who remain hypotensive despite ongoing fluid resuscitation and require support with multiple vasopressors are still often treated with administration of 200 mg of hydrocortisone daily in divided doses. Once vasopressors have been weaned off, corticosteroids may be discontinued as well.5
Tight Glycemic Control
Earlier in the 21st century, practice patterns and randomized trials favored a tighter glycemic control approach (defined as blood glucose 80-110 mg/dl).35 However, a larger international multicenter trial investigating a broader critically ill population subsequently favored a less tight glycemic control approach of (<180 mg/dl) in the ICU.36 Based on these data, a reasonable perioperative goal is a blood glucose <180mg/dl.
Conclusions
With experience in monitoring and resuscitation, the anesthesia provider is ideally suited to care for the septic patient. While a universal set of goals for resuscitation of the septic patient remains elusive, the anesthesia provider has the knowledge and experience to interpret hemodynamic data and apply those principles discussed here to care for these patients. Despite imperfect criteria for defining sepsis, the goals of early recognition, source control, timely antibiotic therapy, and resuscitation remain the foundation for treatment of sepsis.
Todd Dodick, MD, is a Senior Resident in the Department of Anesthesia & Critical Care at the University of Chicago Medical Center. Dr. Dodick has no disclosures.
Steven Greenberg, MD, is Assistant Editor of the Anesthesia Patient Safety Foundation Newsletter and Clinical Associate Professor in the Department of Anesthesiology, University Of Chicago. He is Director of Critical Care Services at NorthShore University HealthSystem. Dr. Greenberg has served as a consultant for CASMED and MERCK. These disclosures are not related to the present article.
Michael O’Connor, MD, is Professor in the Department of Anesthesia & Critical Care at the University of Chicago Medical Center. Dr. O’Connor has no disclosures.
References
- Singer M, Deutschman DS, Seymour CW, Shankar-Hari M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–810.
- Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. NEJM 2015;372:1629–1638.
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, et al. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med 1998;26:1793–1800.
- Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762–774.
- Dellinger RP, Levy MM, Rhodes A, Annane D, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock:2012. Crit Care Med 2013;41:580–637.
- Zhang, D, Micek ST, Kollef MH. Time to appropriate antibiotic therapy is an independent determinant of post-infection ICU and hospital length of stay in patients with sepsis. Crit Care Med 2015;43:2133–2140.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and plea for some common sense. Crit Care Med 2013; 41:1774–1781.
- Cannesson M, Pestel G, Ricks C, Hoeft A, et al. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care 2011;15:R197.
- Michard F, Boussat S, Chemla D, Anguel N, et al. Relationship between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Resp Crit Care Med 2000;162:134–138.
- Canneson M, Le Manach Y, Hofer CK, Goarin JP, et al. Assessing the diagnostic accuracy of pulse pressure variation for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 2011;115:231–241.
- Rivers E, Nguyen B, Havstad S, Ressler J, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. NEJM 2001; 345:1368–1377.
- ARISE investigators and the ANZICS clinical trials group. Goal-directed resuscitation for patients with early septic shock. NEJM 2014;371:1496–1506.
- ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. NEJM 2014;370:1683–1693.
- Mouncey PR, Osborn TM, Power GS, Harrison DA, et al. Trial of early, goal-directed resuscitation for septic shock. NEJM 2015;372:1301–1311.
- Lehman LH, Saeed M, Talmor D, Mark R, et al. Methods of blood pressure measurement in the ICU. Crit Care Med 2013;41:34–40.
- Asfar P, Meziani F, Hamel JF, Grelon F, et al. High versus low-blood pressure target in patients with septic shock. NEJM 2014;370:1583–1593.
- Young JB, Utter GH, Schermer CR, Galante JM, et al. Saline versus Plasma-Lyte in initial resuscitation of trauma patients: a randomized trial. Ann Surg 2014;259:255–262.
- Raghunathan K, Shaw A, Nathanson B, Stürmer T, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med 2014;2014:1585–1591.
- SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. NEJM 2004;350:2247–2256.
- Xu JY, Chen QH, Xie JF, Pan C, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: a meta-analysis of randomized clinical trials. Critical Care 2014;18:702–709.
- Perner A, Haase N, Guttormsen AB, Tenhunen J, et al. Hydroxyethyl starch 130/4.2 versus ringer’s acetate in severe sepsis. NEJM 2012;367:124–134.
- De Backer D, Aldecoa C, Nijmi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med 2012;40:725–730.
- Russell JA, Walley KR, Singer J, Gordon AC, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. NEJM 2008;358:877–887.
- Chawla J, Zia H, Gutierrez G, Katz NM, et al. Lack of equivalence between central and mixed venous oxygen saturation. Chest 2004;126:1891–1896.
- Ladakis C, Myrianthefs P, Karabinis A, Karatzas G, et al. Central venous and mixed venous oxygen saturation in critically ill patients. Respiration 2001;68:279–285.
- Varpula M, Karlsson S, Ruokonen E, Pettilä V, et al. Mixed venous oxygen saturation cannot be estimated by central venous oxygen saturation in septic shock. Intensive Care Med 2006;32:1336–1343.
- Reade MC, Huang DT, Bell D, Coats TJ, et al. Variability in management of early severe sepsis. Emerg Med J 2010;27:110–115.
- Jones AE, Shapiro NI, Trzeciak S, Arnold RC, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized trial. JAMA 2010;303:739–746.
- Nguyen HB, Kuan WS, Batech M, Shrikhande P, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011;15:R229–238.
- Holst LB, Haase N, Wetterslev J, Wernerman J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. NEJM 2014;371:1381–1391.
- Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011;114:695–707.
- Culbertson BH, Sprung CL, Annane D, Chevret S, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med 2009;35:1868–1876.
- Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta-analysis. Crit Care Med 2012;40:2945–2953.
- Sprung CL, Annane D, Keh D, Moreno R, et al. Hydrocortisone therapy for patients with septic shock. NEJM 2008;358:111–124.
- Van den Berghe G, Wouters P, Weekers F, Verwaest C, et al. Intensive insulin therapy in critically ill patients. NEJM 2001;345:1359–1367.
- The NICE-SUGAR study investigators. Intensive versus conventional glucose control in critically ill patients. NEJM 2009;360:1283–1297.


 Issue PDF
Issue PDF